RO flat sheet
The flat sheet reverse osmosis membranes from Alfa Laval are used in an extensive range of processes applied in the food, beverage, dairy, biotech and pharmaceutical industries
The polymeric Alfa Laval flat sheet membranes for reverse osmosis (RO) are made of thinfilm composite based on a unique construction on either polypropylene (PP) or polyester (PET) support material which provides optimum cleaning conditions.
The RO membranes are available in the types: RO90 and RO99 membranes, which are cast on polyester support, with sodium chloride rejections of ≥ 90% and ≥ 98% respectively. The RO98 pHt™ membrane, which has a sodium chloride rejection of ≥ 98%, is cast on polypropylene support and is tolerant to high pH and temperature.
Benefits
- cover a broad spectrum of flux properties, pore sizes, molecular weight cut-off values and rejection capabilities
- available by the metre, as standard 20 x 20 cm sheets or cut into flat sections to fit into all Alfa Laval plate-and-frame module configurations
- delivered with necessary lock and passage rings
- suitable for extensive range of processes
- manufactured by Alfa Laval's own membrane centre
How it works
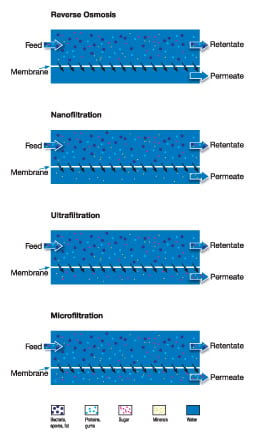
Reverse osmosis (RO)
RO uses the tightest possible membrane in liquid separation. In principle, water is the only material that can permeate the membrane. All other materials (salts, sugars, etc.) will be unable to pass through.
Nanofiltration (NF)
NF is not as fine a separation process as reverse osmosis, and uses membranes that are slightly more open. Nanofiltration allows small ions to pass through while excluding larger ions and most organic components.
Ultrafiltration (UF)
UF involves using membranes in which the pores are larger and the pressure is relatively low. Salts, sugars, organic acids and smaller peptides are allowed to pass, while proteins, fats and polysaccharides are not.
Microfiltration (MF)
In MF, suspended solids, bacteria and fat globules are normally the only substances not allowed to pass through.
Compliances
All materials used for the production of Alfa Laval membranes, in both spiral-wound and flat-sheet membrane designs and configurations, comply with EU Regulation (EC) 1935/2004, EU Regulation 10/2011, EU Regulation (EC) 2023/2006 and FDA regulations (CFR) Title 21. The membranes are thus suitable for use within food and pharmaceutical processing applications.
These compliance also extend to the equipment and fittings related to membrane operations, including items such as plate-and-frame units, element housings and pumps.

